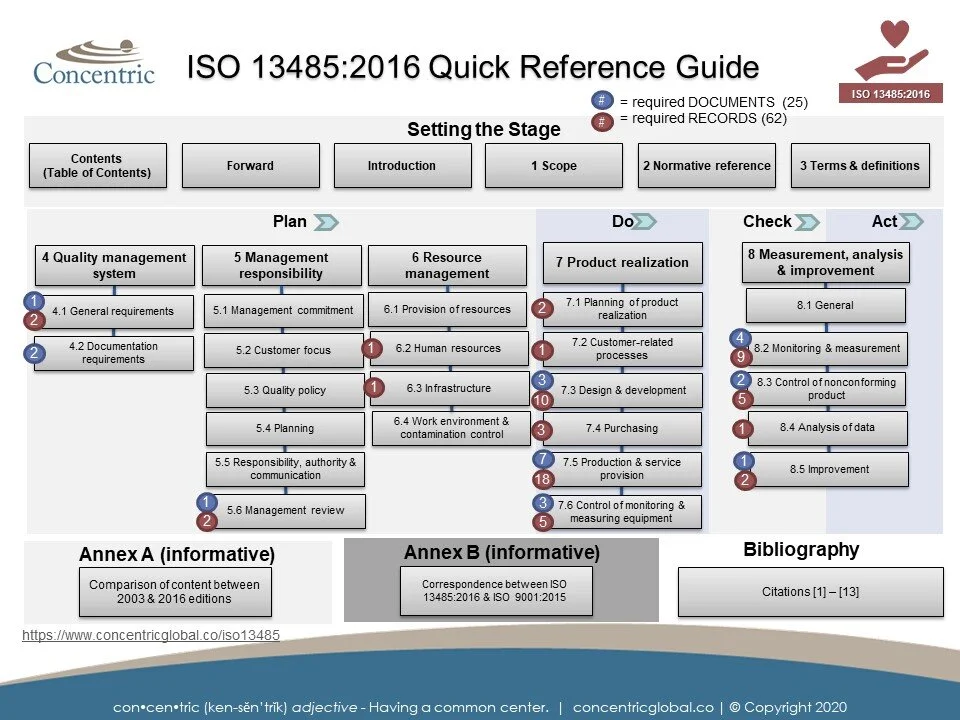
Quality management system requirements based on the ISO 13485 International Standard
Medical Device Series Description: The MD-QMS Series is based on the requirements of the ISO 13485:2016 international quality management system standard. This series provides students with course options ranging from a top management overview to skills necessary for Lead Auditors.
KEY RESOURCES FOR THe HIGHEST QUALITY IN MEDICAL DEVICE PRODUCTION
ISO 13485:2016 International Standard - Purchase from ISO & review accompanying resources
ISO’s Online Browsing Platform (OBP) - Access to online standards, redline changes & more
ISO 13485:2016 Medical Devices: A Practical Guide - Guidance document to the ISO 13485 Standard
ISO 14971:2019 Medical Devices Standard - Application of risk management to medical devices
ISO/TC 210 Committee Homepage - News, project status, guidance & standard revision status
Standards Published by ISO/TC 210 - Quality management and corresponding general aspects for medical devices
PUB100377 - ISO’s free companion guide to the registration scheme for ISO 13485 QMS for Medical Devices
Improving the Safety of Medical Devices by ISO/TC 210 published July 2020
Reducing the Risks of Medical Devices by ISO/TC 210 published December 2019
Overview of ISO 13485 - Medical Devices webinar presented courtesy of PECB
U.S. Food & Drug Administration (FDA) Medical Devices regulations & associated resources
FDA’s Medical Device Database a comprehensive list of data sources in the medical device sector
ISO 10012:2003 Measurement management systems — Requirements for measurement processes and measuring equipment
ISO 11607-1:2006 Packaging for terminally sterilized medical devices — Part 1: Requirements for materials, sterile barrier systems and packaging systems
ISO 11607-2 Packaging for terminally sterilized medical devices — Part 2: Validation requirements for forming, sealing and assembly processes
ISO 14644 (all parts) Cleanrooms and associated controlled environments
ISO 14698 (all parts) Cleanrooms and associated controlled environments — Biocontamination control
ISO 14971:2019 Medical devices — Application of risk management to medical devices
ISO 19011:2018 Guidelines for auditing management systems
IEC 62366-1 Medical devices — Part 1: Application of usability engineering to medical devices
MDSAP Medical Device Single Audit Program Frequently Asked Questions (Version 016 2017-08-22)
FDA’s Code of Federal Reg. Title 21 Dept of H&HS Subchapter H - Medical Devices PART 820
Hours
Price
ISO 13485:2016 Overview for Top Management
4
$295
ISO 13485:2016 Standard Overview
8
$495
ISO 13485:2016 Standard for Internal Auditor & Process Owners
24
$1495
ISO 13485:2016 Lead Internal Auditor
32
$1,850
All of our training programs can be built to meet your individual needs. We have several different delivery methods including:
Public Training: Traditional class room style with students from various companies all over the world meeting in 1 room to review the course materials.
Remote Training: We have the technology to teach any course with students from all over the world in a virtual class room.
Onsite Training: Any of SMEs can come to your facility and teach a course.
One-on-One: This mentorship style of training is great for the student that wants hand on training with any of our SMEs
Self-Paced: We have online training courses that you receive the materials and go as you need.
Do-It-Yourself: This option is great for the organizations that may be looking course materials and have one of their representatives teach the course.
Contact us today to find out which training and methodology is right for you!