Medical Device Series | ISO 13485 QMS








Medical Device Series | ISO 13485 QMS
The QMS-MD Series is based on the requires of the ISO 13485 international environmental management system standard. This series provides students with course options ranging from a top management overview to skills necessary for Lead Auditors.
Download Course Syllabus
-
This training provides in-depth knowledge of standard requirements and industry standards for process design and problem-solving. This course goes beyond compliance requirements and into best practices in auditing techniques and/or problem-solving methods.
EXEMPLAR GLOBAL CERTIFIED: As an additional benefit, you will be Exemplar Global certified and receive a year of access to Exemplar LINK, an exclusive platform that enables you to connect with other auditors, professionals, businesses, and training providers. Exemplar Global benefits include; the opportunity to take advantage of a variety of promotional tools like digital badges and the ability to feature your profile on the Exemplar Global Search Register, free access to professional industry events, free subscription to The Auditor Online, and Exemplar Global’s industry-leading Work Style Assessment development tool.
-
This course provides students with the most comprehensive education within the respective learning track. This training is typically reserved for an organization's most advanced students such as Audit Program Managers, Lean Six Sigma Program Managers, Continuous Improvement/OPEX Leaders, and other top technical leaders.
EXEMPLAR GLOBAL CERTIFIED: As an additional benefit, you will be Exemplar Global certified and receive a year of access to Exemplar LINK, an exclusive platform that enables you to connect with other auditors, professionals, businesses, and training providers. Exemplar Global benefits include; the opportunity to take advantage of a variety of promotional tools like digital badges and the ability to feature your profile on the Exemplar Global Search Register, free access to professional industry events, free subscription to The Auditor Online, and Exemplar Global’s industry-leading Work Style Assessment development tool.
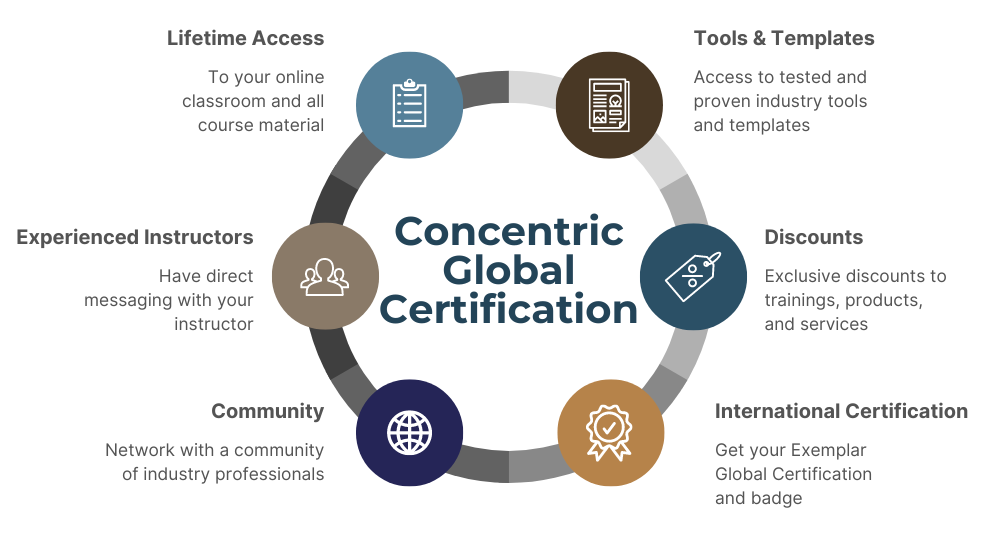
WHEN YOU LEARN WITH CONCENTRIC GLOBAL
BENEFITS OF EXEMPLAR GLOBAL CERTIFICATION
Medical Device QMS classes are held in a series - one module building off of the other - in order to streamline learning and promote diversity among participants. By creating sessions that build off of each other, our classrooms are now made up of organizational leaders, process owners, and an overall better mix of various functions and sectors. This approach not only provides knowledge for a full spectrum of disciplines but improves communication and understanding of standard requirements and process interactions.
If you want to disseminate the design, development, and maintenance of each process within your organization's operations, this approach will be a game-changer. If you're looking for value, better buy-in, and creating a more productive Medical Device QMS, this series is for you.
Benefits to Leadership & Process Owners: Knowledge is power. Learning not only WHAT is required but HOW to satisfy these requirements in a way that works for you (vs. shoving your business into the "ISO Box"), management system implementation is properly distributed throughout the organization. Learn how to become your own Management System Architect by mastering the processes you manage!
Benefits to 1st & 2nd-party Auditors: The auditing portion of our Medical Device QMS series grows from the knowledge needed for new auditors to Audit Program Managers. Depending on your role in auditing activities, this series caters to the full spectrum of auditor responsibilities from process auditing to audit program management. Using the framework of ISO 19011 Guidelines for management systems auditing and IATF Oversight Communications, this course will teach you the fundamental tools necessary to conduct audits using the process approach while providing vital strategic information to leadership and process owners. Case studies and audit scenarios make this course fun and interesting. If you are looking for a course that will add more value to your internal auditing program with real business impact, you have found it with this series.
SOME OTHER BENEFITS OF OUR ONLINE TRAINING:
Self-paced training platform
Facilitator bundles
Key links to applicable standards & support documents
Case studies & best practices
Articles (ISO updates, blogs, etc.)
Online community & discussion forum
Digital tools & templates
Access to Ask the Expert